 ISSN: 1658-807X
ISSN: 1658-807X EISSN: 1658-8088
Journal of Biochemical and Clinical Genetics
The Journal of Biochemical and Clinical Genetics (JBCGenetics) is a peer-reviewed, open-access medical journal. It is the official journal of the Saudi Society of Medical Genetics.Are you facing technical difficulties with submission? Our support team will assist you. Please send an email to: Anastasiia.Pavlenco@sofiafields.com
Already submitted an article?

Articles
A novel biallelic frameshift variant in MYO15A causing nonsyndromic hearing loss in Saudi family
Non-syndromic intellectual disability and cataract in a patient with dual molecular diagnosis of SRD5A3 and PITX3-related diseases
Genetic characterization and clinical correlation in a cohort of Turkish patients with immunodeficiency: insights from whole exome sequencing
CFTR interactome may impact gastric cancer: an in silico system-level coexpression analysis
Message from Editor in Chief
A warm welcome to the Journal of Biochemical and Clinical Genetics! We're thrilled to share cutting-edge research, innovative discoveries, and expert insights in Genetics field with you. Our journal is dedicated to advancing the fields of biochemical and clinical genetics, fostering collaboration, and promoting scientific excellence. We invite you to explore our latest articles, reviews, and research papers, and novel case reports . Let's unravel the complexities of genetics and genomics together, driving progress and improving human health. Thank you for being part of our scientific community!"
News
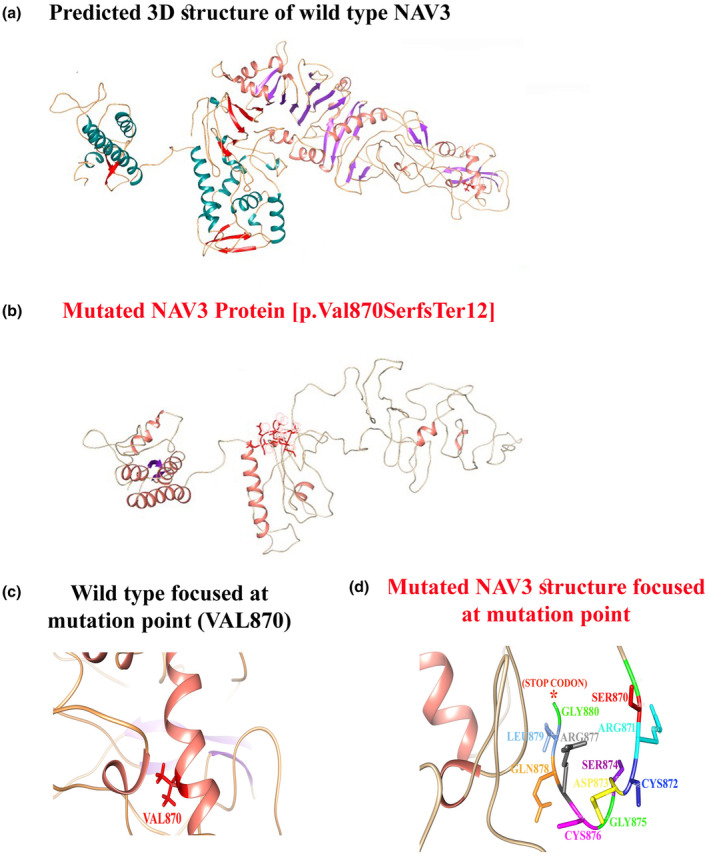
Breaking Discovery: NAV3 Gene Linked to a Novel Neurodevelopmental Disorder (NEDUA) – OMIM #621182
- Gene: NAV3 (Neuron Navigator 3), crucial for neuronal morphogenesis and axonal guidance.
- Clinical Features: Patients present with global developmental delay, poor or absent speech, dysmorphic facies, microcephaly, hypotonia, and additional neurodevelopmental features.
- Inheritance: Autosomal recessive, with both homozygous and compound heterozygous variants identified in affected families.
- Impact: This discovery provides much-needed answers for families worldwide and opens new avenues for research into targeted therapies and genetic counseling.
Announcements

Technical Alert
Dear authors, reviewers, and visitors, The Journal of Biochemical and Clinical Genetics (JBCGenetics) is currently in the process of migrating to a new server and a new online journal submission and publishing system (epublisyst.com). You may experience temporary site outages, broken links, or other technical issues. We kindly ask for your patience during this transition, and we will be back online as soon as possible.
If you had submitted a manuscript through our previous submission system (eJmanager), you can continue to check for updates by logging into the previous journal submission system:
https://www.ejmanager.com/my/jbcg/
Reviewers who accepted to review via our previous journal system (eJmanager), may log in via following link:
https://www.ejmanager.com/reviewers/index.php?isl=login
Thank you for your understanding and continued support.

